Concrete Deterioration: Carbonation & Chloride Ion Attack
- Reinforced concrete (RC) is concrete embedded with reinforcing steel bars (rebars), steel mesh, etc.
- RC deteriorates due to several reasons, including the corrosion of rebars.
- Carbonation and chloride ion attack are 2 of the most commonly discussed phenomena for RC deterioration.
- Carbonation & chloride ion attacks cause the alkalinity of concrete to decrease, thus resulting in an unstable passivating layer. This in turn causes rebars to corrode.
What is Reinforced Concrete?
Concrete is one of the most used construction materials due to its exceptional durability & versatility. Generally, all concrete is reinforced to support larger weights and critical loads.
Reinforced concrete (RC), concrete embedded with steel bars, works on the principle that the two materials act together to resist forces acting on a structure. The reinforcing steel (rods, bars, or mesh) would absorb tensile stresses, whereas concrete absorbs compressive stresses. The high alkalinity of concrete (pH between 12.5 – 13) also creates a passivating layer around the reinforcing steel bars (rebars), which protect them from corrosion.
However, deficiencies in material, design, construction practices and surrounding conditions will affect the reinforced concrete’s service life. The deterioration of reinforced concrete is resulted by a combination of factors.

Reinforcing steel embedded in concrete
Deterioration of Reinforced Concrete
- Deterioration is described as a process of degeneration in quality to an inferior state of a material over a period of time.
.png)
(Image from ResearchGate)
Carbonation Induced Deterioration
- Carbonation is a chemical reaction between carbon dioxide gas (CO2) with portlandite (CaOH2) within the cement to form calcite (CaCO3). Carbon dioxide gas also dissolves in water to form carbonic acid (H2CO3), which attacks the concrete and leads to the depletion of hydroxyl ions. The following shows the chemical process of concrete carbonation:
- This reduces the alkalinity of concrete to a pH level below 9, which results in an unstable passivating layer around reinforcement. With sufficient oxygen and water present in the surrounding area of rebars, this low alkalinity condition would cause general corrosion to occur effortlessly.
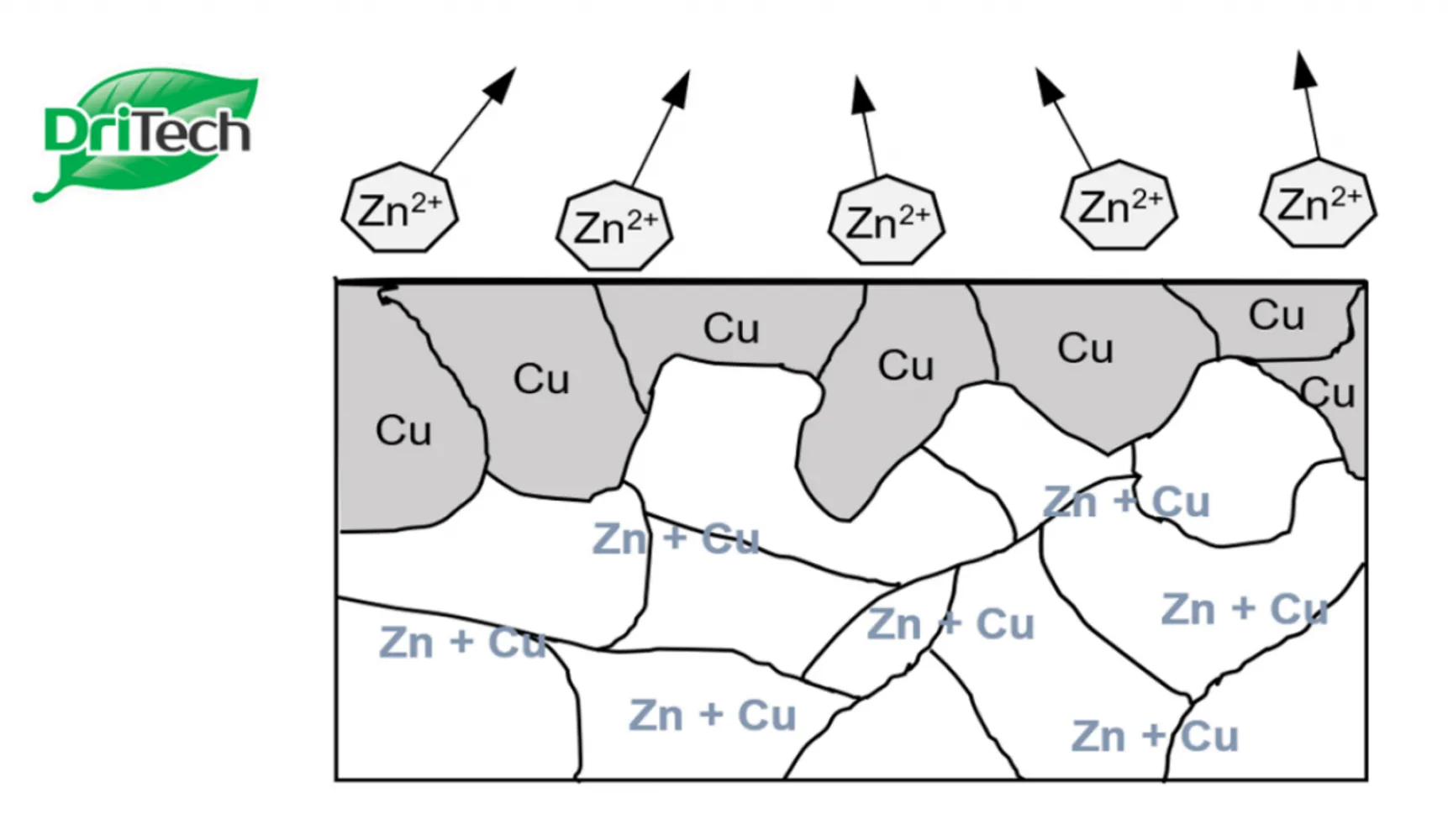
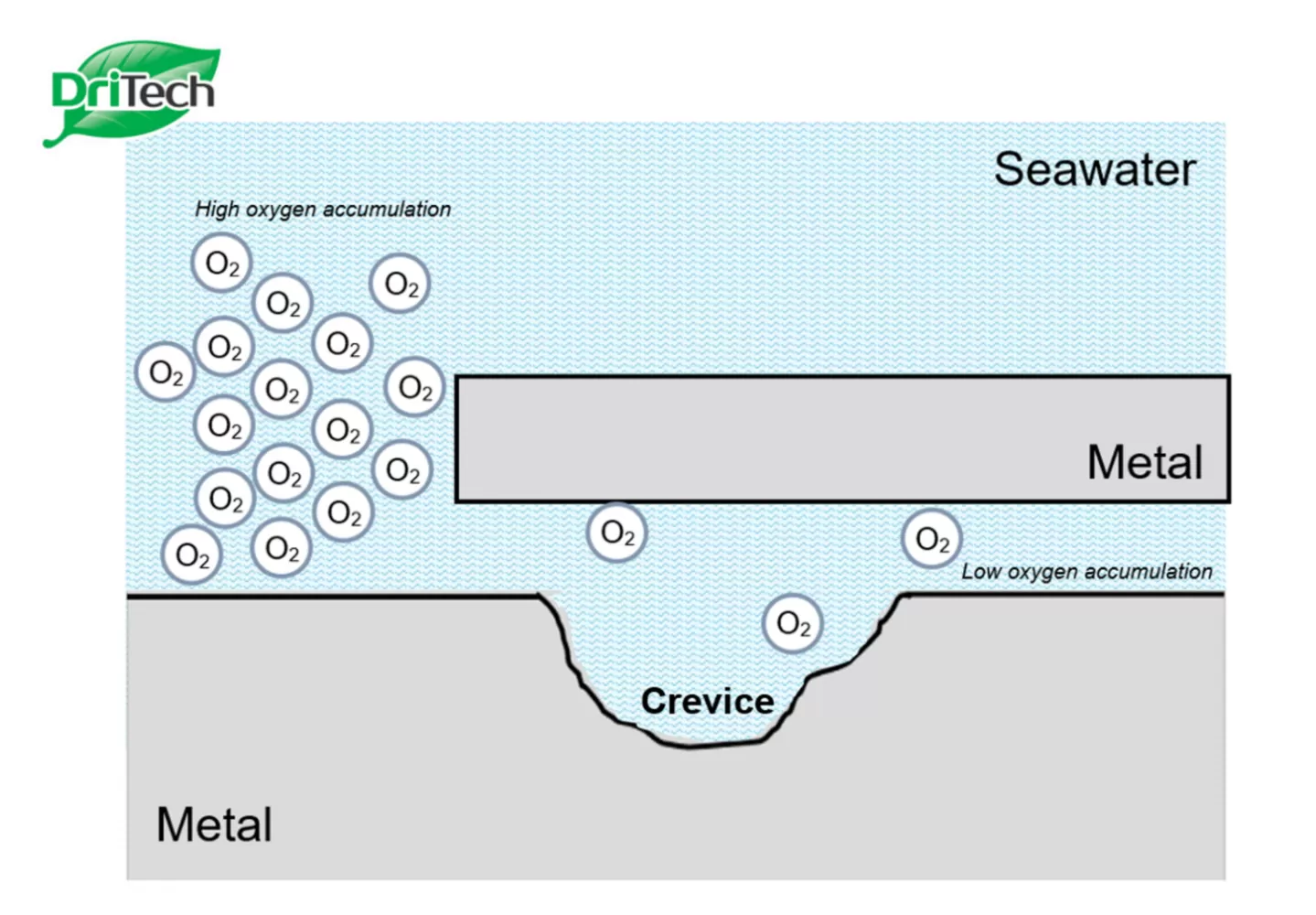
- The attack of chloride ions can occur either from inside the RC (from the aggregates used) or through the ingress of chloride ions from the surrounding. For example, a marine environment, an environment with soils rich in chloride deposits, or seawater wetting are typical conditions where chloride attack could occur at an aggressive rate. The process of chloride attack is shown in the following reaction:
- The newly formed ferrous chloride then reacts with water to form ferrous hydroxide and hydrochloric acid, which leads to lowering of pH level. The passivating layer, normally maintained by the alkaline environment, will be disrupted and corrosion is initiated in the presence of sufficient chloride ions, water, and oxygen.
Hence, as chloride content in concrete increases, oxidation is able to take place with the creation of ferrous ions. The presence of hydrochloric acid will generate a cycle for continual corrosion with the balance chloride ions.
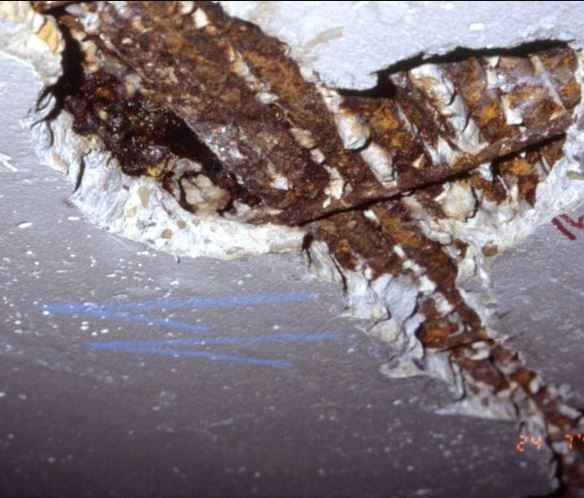
Concrete Spalling
- The two mechanisms stated above will result in the creation of rust. As rust occupies larger volume than steel, it will exert an outward pressure, which causes the surrounding concrete to crack. These leads to much easier penetration of carbon dioxide and chloride ions, which further speeds up the corrosion process. Eventually, this will lead to failure of the structure.
.png)
Process of concrete spalling (Image from Civil Engineering Forum)
Hence, proper design & maintenance must be done on RC structures to prevent any detrimental effects. An effective protective coating system can be introduced to prevent carbonation & chloride attack from occurring by forming a barrier on the surface. This can block off the pores of concrete to prevent diffusion of any unwanted material. International standards, such as EN1504, can be used as a guide for concrete protection, restoration and protective coating selection for structures. (Head over here to read more on EN1504 & Protective Coating Systems)
Please contact us for more information on appropriate protective coating systems for your structures.
by Isaac Chin
[28 August, 2021]
15 Dec 2022






.jpg)